OUR RESPONSE
Planetary Genetics and Mimigen Technologies are taking action to respond to the current Coronavirus (COVID-19) pandemic. Our team consists of highly experienced biotechnology professionals who have delivered some of the most advanced diagnostic testing platforms, including one of the primary rapid response detection platforms being employed throughout the world in blood banks such as the American Red Cross and Japanese Red Cross, large reference laboratories including Quest, LabCorp, etc., hospital systems, as well as, military and public health laboratories.
The information below is being provided as background for government leaders, healthcare workers and the general public as we respond in this time of crisis to the COVID-19 pandemic. The framework presented here is recommended as context for advancing testing strategies during this time of rapidly changing circumstances and evolving information streams. We anticipate strategies will evolve as resources are made available and information is better disseminated.
The information below is being provided as background for government leaders, healthcare workers and the general public as we respond in this time of crisis to the COVID-19 pandemic. The framework presented here is recommended as context for advancing testing strategies during this time of rapidly changing circumstances and evolving information streams. We anticipate strategies will evolve as resources are made available and information is better disseminated.
ENVIRONMENTAL DIAGNOSTICS
Our team is advancing environmental diagnostic capabilities to curtail the spread of COVID-19 by utilizing molecular assays in sewer surveillance to serve as a rapid-response detection platform for future outbreaks.
MEDICAL DIAGNOSTICS
LINK TO PDF
published 20 MAR 2020 | updated 15 OCT 2020
published 20 MAR 2020 | updated 15 OCT 2020
Concurrently, we are focused on advancing COVID-19 (SARS-CoV-2) quantitative serological testing capacity for protective neutralizing antibodies as part of our overall health mission. We are specifically designing next-gen quantitative diagnostics to respond to the COVID-19 pandemic and serve as a platform for measuring patient immune profiles following vaccination or exposure to viral variants.
BACKGROUND
Antigen, molecular and serological diagnostic testing are medical countermeasures that enable the mitigation and containment of infectious disease outbreaks and epidemics such as the novel Coronavirus (SARS-CoV-2).
ANTIGEN TESTING is a rapid method of testing used for direct detection of an infecting organism by recognizing specific proteins from the organism present in a patient’s blood or nasal/oral fluids. Antigen tests are less sensitive than molecular tests so are at higher risk of false-negative results and a subsequent molecular test may be needed to verify negative antigen test results.
MOLECULAR TESTING detects and identifies the infecting organism by the presence of its DNA or RNA. It is a direct detection of the organism and usually enables detection of the presence of microbial or viral nucleic acids much earlier in the course of an infection. The empirical sensitivity of most molecular methods, especially nucleic acid amplification tests (NAAT), also tends to decrease the time from infection to a positive assay. The increased sensitivity comes with the risk of false-positives and requires highly skilled operators to reduce the possibility of cross-contamination.
SEROLOGICAL TESTING (antibody) for infectious disease diagnosis has been available since the early and middle part of the past century and is a cost-effective way to screen a large population for both symptomatic and asymptomatic people. Serological testing is based upon the ability of an individual to mount an effective humoral (antibody) immune response to a pathogen. The test is not a direct detection of the organism but measures a person’s immune response (seroconversion status) to the pathogen. Therefore, serological tests can identify people who went through an infection, recovered, and cleared the virus from their bodies.
As we look ahead to the lead time for development of vaccine and therapeutic interventions, we recognize the need for and synergies between antigen, molecular and serological testing. These varied testing approaches can play an important in mitigating the spread of the SARS-CoV-2 pathogen. The diagram below conveys patient testing journeys to monitor their status across the three testing modalities.
ANTIGEN TESTING is a rapid method of testing used for direct detection of an infecting organism by recognizing specific proteins from the organism present in a patient’s blood or nasal/oral fluids. Antigen tests are less sensitive than molecular tests so are at higher risk of false-negative results and a subsequent molecular test may be needed to verify negative antigen test results.
MOLECULAR TESTING detects and identifies the infecting organism by the presence of its DNA or RNA. It is a direct detection of the organism and usually enables detection of the presence of microbial or viral nucleic acids much earlier in the course of an infection. The empirical sensitivity of most molecular methods, especially nucleic acid amplification tests (NAAT), also tends to decrease the time from infection to a positive assay. The increased sensitivity comes with the risk of false-positives and requires highly skilled operators to reduce the possibility of cross-contamination.
SEROLOGICAL TESTING (antibody) for infectious disease diagnosis has been available since the early and middle part of the past century and is a cost-effective way to screen a large population for both symptomatic and asymptomatic people. Serological testing is based upon the ability of an individual to mount an effective humoral (antibody) immune response to a pathogen. The test is not a direct detection of the organism but measures a person’s immune response (seroconversion status) to the pathogen. Therefore, serological tests can identify people who went through an infection, recovered, and cleared the virus from their bodies.
As we look ahead to the lead time for development of vaccine and therapeutic interventions, we recognize the need for and synergies between antigen, molecular and serological testing. These varied testing approaches can play an important in mitigating the spread of the SARS-CoV-2 pathogen. The diagram below conveys patient testing journeys to monitor their status across the three testing modalities.
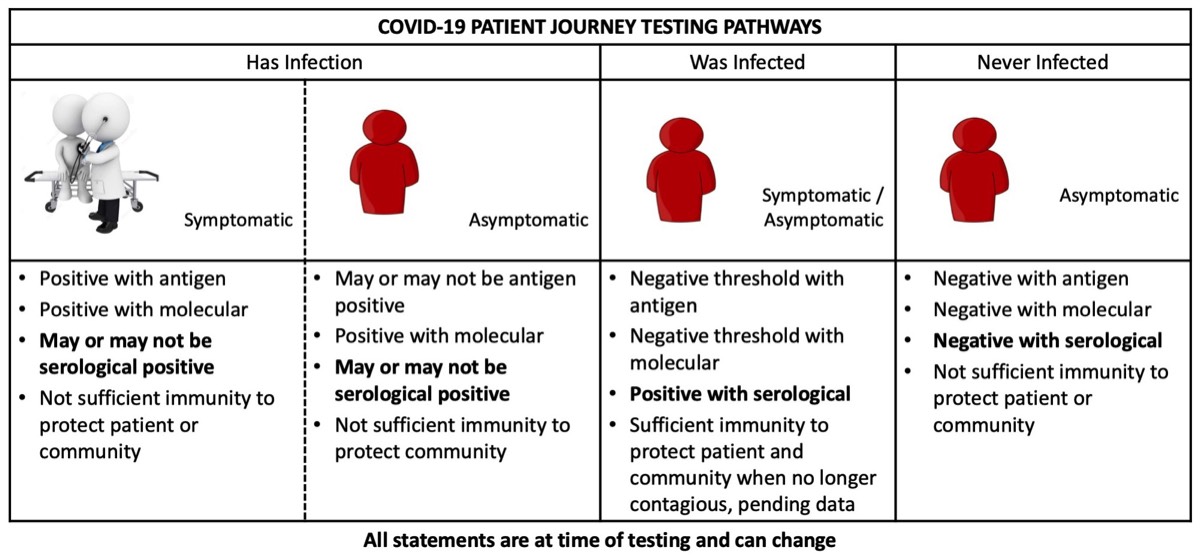
Molecular testing is extremely sensitive and can detect the presence of a microbe much earlier in the course of an infection. These tests can detect low levels of the pathogen before there is an immune response. Molecular tests are considered high complexity with a high cost per test, and as such, it can be very expensive to screen populations utilizing this technology. When time has elapsed and a patient has likely cleared the virus, it is too late to confirm an infection using molecular testing thus preventing a population-based method to track who has had an infection.
Serological testing is a cost-effective way to screen an entire population to identify disease exposure and establish a cutoff for when people who have had the disease are no longer contagious. Serological screening will allow first-responders and healthcare workers to return to the front lines and allow family members to return to care giving once they are no longer infectious. A quantitative neutralizing antibody test could be synergistically coupled to antigen testing of blood to determine current status of infection, as well as, provide measures of protective immunity. Understanding the status of people's immunity will be an immediate stopgap measure prior to widespread vaccination availability and could help prioritize the order of patients offered vaccination. Upon availability of vaccines, serological testing will serve to screen patient’s seroconversion status and determine vaccination efficacy. Finally, serological testing has potential to reduce the number of molecular diagnostics for viral testing used in repeat testing of patients.
Serological testing is a cost-effective way to screen an entire population to identify disease exposure and establish a cutoff for when people who have had the disease are no longer contagious. Serological screening will allow first-responders and healthcare workers to return to the front lines and allow family members to return to care giving once they are no longer infectious. A quantitative neutralizing antibody test could be synergistically coupled to antigen testing of blood to determine current status of infection, as well as, provide measures of protective immunity. Understanding the status of people's immunity will be an immediate stopgap measure prior to widespread vaccination availability and could help prioritize the order of patients offered vaccination. Upon availability of vaccines, serological testing will serve to screen patient’s seroconversion status and determine vaccination efficacy. Finally, serological testing has potential to reduce the number of molecular diagnostics for viral testing used in repeat testing of patients.
ASSUMPTIONS
Until vaccines and therapeutic treatments are ready to be deployed, testing strategies are the only way to mitigate the spread of infection during this pandemic.
- Vaccines will be available to the general public first half of 2021, purportedly.
- Molecular test deployment will scale across high-throughput screening testing sites at existing large reference and public health laboratories.
- Linked testing of pooled patient samples should be evaluated for economies of scale, pending data.
- It should be expected that this outbreak will be multi-phasic over seasons, meaning there is the potential for subsequent waves to be more severe among the vulnerable population.
- Ancillary assay equipment and components required to run some tests may not be available thus hindering testing.
- With proper resources, it can take 6-8 months to develop quantitative serological assay chemistries for neutralizing antibody testing.
- Domestic response capabilities will shift to international global health response in the interest of national and global security.
PUBLIC HEALTH TESTING STRATEGIES
Under the current circumstances of the COVID-19 pandemic and given limited supplies of diagnostic testing kits, it is important to utilize our precious testing resources in a practical way to implement testing in the context of strained supply chains. The response outlined below is specific to COVID-19 global pandemic and not necessarily the general order of smaller outbreak and epidemic responses. Deployment of recommended resources will be limited until testing capacity exceeds demand. One can expect in the time of pandemic there will be local breakdowns of capacity and shortages of critical components to render testing and care.
CHALLENGE: Employ ANTIGEN, MOLECULAR, and QUANTITATIVE SEROLOGICAL TESTING
- Use of antigen or molecular testing alone presents a PUBLIC HEALTH OBSTACLE since these tests cannot identify people who went through an infection, recovered, and cleared the virus from their bodies. If antigen or molecular testing were to be used exclusively, repeat testing on the same patient would be required because the testing is qualitative and is not a quantitative viral load with an established clinical endpoint.
- We have a NEED for a test to be available on the market to determine when antigen or molecular testing is no longer required because immunity is established in patients by the presence of sufficient protective and neutralizing antibodies.
- We have a CRITICAL NEED for serological testing to know a patient’s seroconversion status in order to (1) avoid unnecessary repeat patient testing to conserve antigen and molecular test kits and (2) facilitate return of healthcare workers, first responders, and families to the front lines of care giving as soon as they are no longer infectious (3) cost effectively identify asymptomatic carriers who are still contagious.
PHASE 1A: MOLECULAR DIAGNOSTIC viral testing of symptomatic population
- Molecular testing of nasal and oral swabs
- Automated molecular diagnostics infrastructure is present in major reference laboratories (LabCorp, Quest), as well as, state and local public health laboratories. Major diagnostics biotech companies (including early testing providers Roche, Thermo Fisher Scientific, Hologic) should meet capacity requirements for scaling molecular tests during this crisis.
- BENEFIT: Testing identifies symptomatic infected persons in clinical settings.
- LIMITATIONS: Molecular diagnostic testing is not a point-of-care test. Repeat testing may be required until either vaccine administration or multiple follow-up tests indicate seroconversion. Additionally, the virus may mutate and this type of testing may not detect all variants of the mutated virus. Molecular testing is expensive, time-consuming, and will be cost-prohibitive at certain scales.
PHASE 1B: QUALITATIVE ANTIBODY (SEROLOGICAL) TESTING of symptomatic and asymptomatic population
- Serological (blood) tests deployed that measure the level of auto-antibodies in patients against SARS-CoV-2 (COVID-19) virus (i.e. presence of protective immunity). These tests can be used with automated instrumentation in reference laboratories for high-throughput screening and/or at the point-of-care/concern with various device formats.
- BENEFITS: Allows patients to know immunity status. Enables healthcare workers, first responders, and family members to return to front the lines of care giving with indication of some protective immunity. Will help triage patients in greatest need of vaccination. A cost-effective way to identify asymptomatic carriers who are still contagious.
- LIMITATIONS: The requirements for seroconversion, a measure of neutralizing antibodies, to provide full protective immunity are still being determined. It is possible to miss infected patients because the body has not generated a significant and proper immune response. Virus may mutate, and serological tests may not detect all variants depending on mutations.
PHASE 2A: ANTIGEN TESTING of symptomatic and asymptomatic population
- Viral testing of nasal swabs with potential for sputum or blood specimens
- BENEFIT: Testing identifies symptomatic infected persons in clinical settings and a percentage of asymptomatics in non-clinical settings. Antigen testing is rapid and can be administered at point-of-care. Antigen testing could be synergistically coupled to quantitative neutralizing antibody testing of blood to determine current status of infection, as well as, provide measures of protective immunity.
- LIMITATIONS: Antigen testing is faster and less expensive than molecular testing but has increased risk of false-negatives, which may require follow-up molecular tests to confirm results. Repeat testing may be required until either vaccine administration or multiple follow-up tests indicate seroconversion. Additionally, the virus may mutate and this type of testing may not detect all variants of the mutated virus.
PHASE 2B: MOLECULAR DIAGNOSTIC viral screening of asymptomatic population
Expand molecular testing to larger population showing no current symptoms (asymptomatic).
- Viral testing of nasal swabs with potential for sputum or blood specimens
- Automated molecular diagnostics infrastructure is present in major reference laboratories (LabCorp, Quest), as well as, state and local public health laboratories. Major diagnostics biotech companies (including early testing providers Roche, Thermo Fisher Scientific, Hologic) will meet capacity requirements at a to be determined time when testing of symptomatic patients is well-managed.
- BENEFIT: Testing identifies asymptomatic infected persons within the general population.
- LIMITATIONS: Repeat testing may be required until vaccine administration or testing indicates seroconversion. Virus will mutate and tests may not detect all variants depending on mutations. Molecular testing is expensive, time-consuming, and will be cost-prohibitive at certain scales.
NEXT TESTING INNOVATION PHASE: QUANTITATIVE NEUTRALIZING ANTIBODY (SEROLOGICAL) TESTING of symptomatic, asymptomatic, and vaccinated populations
Quantitative serological antibody tests for seroconversion for SARS-CoV-2 do not exist currently in the public domain. However, the core technologies to deliver a quantitative chemistry solution to provide the next generation of immunoassay testing is available. Expansion of serological testing from Phase 1 qualitative testing to more precise quantitative testing would be impactful. Qualitative COVID-19 serological testing does not measure the precise quantity of SARS-CoV-2 antibodies and is used for screening purposes. Quantitative serological testing measures the titer of neutralizing antibodies against the novel virus causing COVID-19 allowing for detection at timepoints indicating a person has gone through an infection, recovered, and cleared the virus from the body.
- Blood tests to measure antibodies against SARS-CoV-2 (COVID-19) virus and determine seroconversion, which is to say measures of protective immunity against the pathogen.
- These tests can be used with automated instrumentation in reference laboratories for high-throughput screening and/or at the point-of-care/concern with various device formats.
- BENEFITS: Allows patients to know seroconversion status. Enables healthcare workers, first responders, and family members to return to front the lines of care giving with indication of some protective immunity. Will help triage patients in greatest need of vaccination. Tests can also be used to determine efficacy of vaccination.
- LIMITATIONS: Seroconversion requirements for full protective immunity to be determined. Virus will mutate and tests may not detect all variants depending on mutations.
ADDITIONAL RESOURCES
- CDC Coronavirus (COVID-19) Information and Resources
- Johns Hopkins University Center for Systems Science and Engineering Coronavirus COVID-19 Global Cases
- ACOER Coronavirus (COVID-19) Tracker (daily tracking and visualizations of the pandemic)
- COVID-19 Testing Capacity and Action Tracker
- Coronavirus Test Tracker: Commercially Available COVID-19 Diagnostic Tests (in use or under regulatory evaluation)
For more information regarding Planetary Genetics and Mimigen Technologies COVID-19 Diagnostics Development Program, please contact info@planetary-genetics.com.
Learn more here about WHY US?
Learn more here about WHY US?